
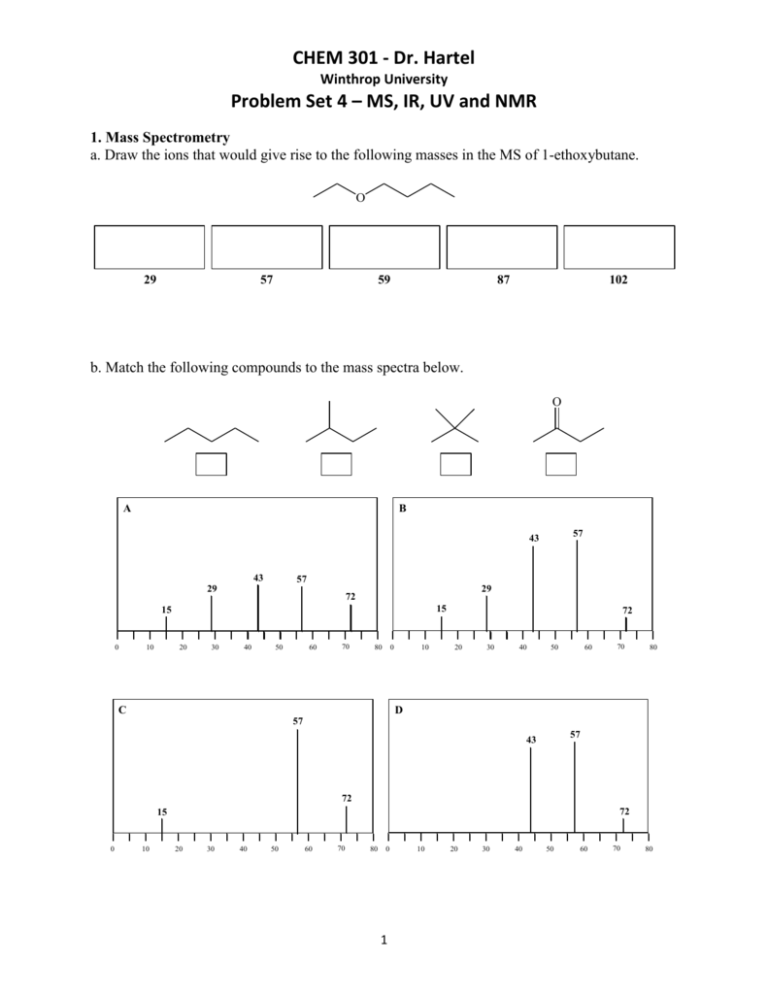
Some elements have more than one isotope of high natural abundance ( e.g. The signals with lower m/z are fragment ions and can provide some structural information.Įxample: The mass spectrum of butanone would be predicted to have a signal at the following m/z values:Įach fragment recorded in the mass spectrum registers the specific isotopes of the various elements present. The mass spectrum of a compound typically shows a number of signals and the peak at highest m/z ( molecular ion) usually corresponds to the mass of the whole molecule. The ions are characterised by their mass ( m) to charge ( z) ratio ( m/z). The cations are accelerated through an electric field into a magnetic field where they are deflected. The molecular ions (M +.) may break down into fragment ions or daughter ions (m +). This causes electrons to be ejected from molecules in the sample, leaving them as positively charged cations – the molecular ion or parent ion. A small sample of the compound is vaporised under very low pressure and high temperature and the vapour is irradiated with a beam of high energy electrons (4000 – 6000 kJ mol -1). Mass spectrometry is used to determine the molecular mass of an organic compound. Benzene (C 6H 6) and acetylene (C 2H 2) both have the empirical formula CH, but different molecular masses and molecular formulas. In order to determine the molecular formula of a compound, the molecular mass of that compound is required. Quantitative analysis for C, H and N in an organic compound is routine and if heteroatoms such as the halogens, S or P are absent, oxygen is usually assumed to make up the difference to 100%.Įxample:Ě compound returns the following analysis: C = 54.55%, H = 9.09%. chromatography (solids, liquids, gases).It may be conducted by chemical methods or physical methods Isolation and purification of an unknown compound is an essential first step to identification.
Assemble the formula of the molecule with the correct constitution and stereochemistry.Determine the elements present (empirical formula).The major steps involved in determining the structure of an unknown compound are: Reference: McMurry Ch 13 George et al Ch 3.1, 3.2


 0 kommentar(er)
0 kommentar(er)
